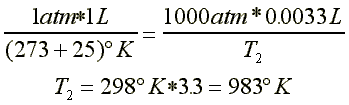The Ideal Gas Equation
The three historically important gas laws derived relationships between two physical properties of a gas, while keeping other properties constant:
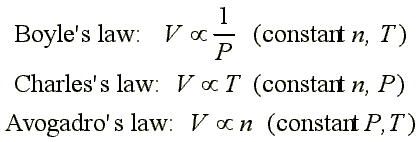
These different relationships can be combined into a single relationship to make a more general gas law:
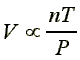
If the proportionality constant is called 'R', then we have:
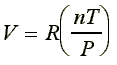
Rearranging to a more familiar form:
![]()
This equation is known as the ideal-gas equation
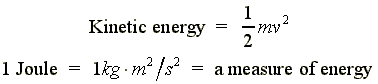
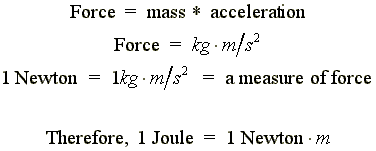
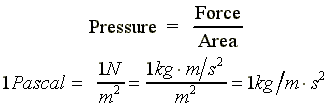
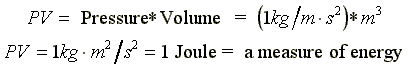
|
Values for the gas constant R |
|
|
Units |
Value |
|
L atm/mol K |
|
|
cal/mol K |
|
|
J/mol K |
|
|
m3 Pa/mol K |
|
|
L torr/mol K |
|
Example:
If we had 1.0 mol of gas at 1.0 atm of pressure at 0°C (273.15 K), what would be the volume?
PV = nRT
V = nRT/P
V = (1.0 mol)(0.0821 L atm/mol K)(273 K)/(1.0 atm)
V = 22.41 L
The molar volume of an ideal gas (any ideal gas) is 22.4 liters at STP
Example: Nitrate salts (NO3-) when heated can produce nitrites (NO2-) plus oxygen (O2). A sample of potassium nitrate is heated and the O2 gas produced is collected in a 750 ml flask. The pressure of the gas in the flask is 2.8 atmospheres and the temperature is recorded to be 53.6 °C.
How many moles of O2 gas were produced?
PV = nRT
n = PV/RT
n = (2.8 atm * 0.75 L) / (0.0821 L atm/mol K * (53.6 + 273)K
n = (2.1 atm L) / (26.81 L atm/mol)
n = 0.078 mol O2 were produced
Relationship Between the Ideal-Gas Equation and the Gas Laws
Boyle's law, Charles's law and Avogadro's law represent special cases of the ideal gas law
PV = nRT
PV = constant
P = constant * (1/V)
P ![]() 1/V
(Boyle's law)
1/V
(Boyle's law)
PV = nRT
V = (nR/P) * T
V = constant * T
V ![]() T
(Charles's law)
T
(Charles's law)
PV = nRT
V = n * (RT/P)
V = constant * n
V ![]() n
(Avogadro's law)
n
(Avogadro's law)
PV = nRT
(PV)/T = nR = constant
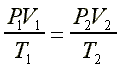
Example:
A 1 liter sample of air at room temperature (25 °C) and pressure (1 atm) is compressed to a volume of 3.3 mls at a pressure of 1000 atm. What is the temperature of the air sample?